Putting CAR T Therapies to the Test: A Case Study on the Saver of a 20-year-old Woman from Brain Damage
People with other disorders could benefit from hyper-personalized therapies. Scientists and regulators need to work together to make sure the benefits are spread.
The chatter is different today. More than 30,000 people with cancer have received engineered CAR T immune cells in the US. CAR-T therapy is being tested for other conditions, including some severe autoimmune disorders. As for commercial success, in 2023, CAR T cells earned biotechnology companies US$8.4 billion worldwide.
Both approaches are fraught with challenges. As in the early days of CAR-T therapy, many of them are not scientific. Researchers can help shepherd therapies to the people who need them by guiding regulators and developing flexible platforms.
Researchers have been chasing vaccines that could help the immune system fight off tumors. Companies now have the ability to sequence portions of a person’s tumours and select which parts will be visible to the immune system. The mRNA molecules corresponding to those regions are synthesized, then encapsulated in fatty particles and injected — much like mRNA COVID-19 vaccines. From start to finish, the process takes as little as a month.
For-profit companies can’t be relied on to develop platforms for CRISPR-based therapies if the market is small. Researchers are trying to develop platforms for this type of therapy. More should be done to join, or the chance to use genome editing to correct genetic disorders that are often rare will be wasted.
The team of researchers were racing to come up with a solution that would save the 20-year-old woman from becoming brain damaged from the toxic chemicals in her cells. It was an approach that hadn’t been done before and had a number of reasons why it wouldn’t work.
The team made rapid progress. The researchers were maybe six months away from being ready to give Uditi the therapy, Ghosh told her parents over breakfast at their home outside New Delhi last June. Even so, Uditi’s mother was not satisfied. Work faster, she urged him.
The work that they did on this project could help future attempts to develop genome-editing therapies for genetic conditions, as the family wanted them to do, despite the fact that Uditi’s illness had already progressed past the point at which the therapy could offer a notable benefit.
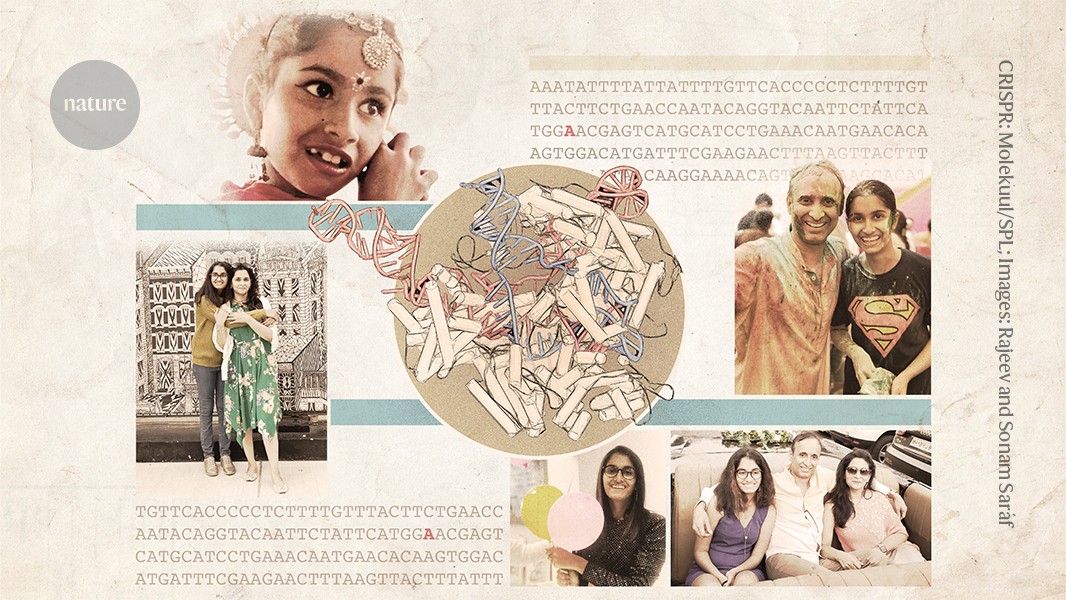
As a young girl, Uditi was always in a hurry. She would blow any excuse for a party to celebrate her mother’s birthday, even if it meant getting ready hours before everyone else. She welcomed everyone with hugs and kisses and entertained the crowd with her dancing.
There was no sign of trouble for the first nine years. And when it began, it was just a flicker — a few seconds here and there, when Uditi would zone out.
Her mother was unsure if she should worry, as she would switch back on as if nothing had happened. But then Sonam saw nine-year-old Uditi drop a camera on the floor and become confused as to why it was no longer in her hand. A mother had a hunch that something was wrong.
Uditi was sent to a school for people with disabilities after the three moved to upstate New York. Her seizures intensified, and frequent muscle spasms made it hard for her to walk or drink from a glass. Her bright personality was getting dimmer. The Sarafs discussed experimental treatments with Uditi’s new physician, epilepsy specialist Orrin Devinsky at NYU Langone Health in New York City. One of the options was genome editing. The idea was seized on by Rajeev.
The Saraf studied what online could teach them and tried a number of interventions, including an Indian version of a ketogenic diet. “We shopped for doctors. Sonam says they hunted for gods and Uditi worsened her condition slowly.
One of the most important reagents was foreign antibodies that could be used to see the brain and its tangles. Few researchers use such antibodies, and the supply was uncertain. The team decided to ask Miranda in Rome to share the ones that she had developed the fastest way to get reliable antibodies. She gladly did. She says it was a desperate approach. For me, the priority is to help as much as I can.
The symptoms of FENIB can be similar to dementia, so they are usually seen late in life. Elena Miranda, a cell biologist at the Sapienza University of Rome, runs the world’s only lab that focuses on the disease. She says that it’s possible that many cases of FENIB go unreported because physicians do not often sequence the genomes of older adults with dementia.
Severe forms of FENIB are rare and strike early. Miranda has known of only three other people with the same mutation that Uditi had. She says this form of the disease is very aggressive.
Uditi’s disease was caused by a mutation that converts a single DNA base from a ‘G’ to an ‘A’. A variation on CRISPR genome editing, called base editing, could theoretically correct exactly this kind of mutation (see ‘Precision gene repair’).
Devinsky also emphasized the difficulties. At that time, base editing — which was first reported in 2016 — had never been tested in a clinical trial. The technique requires shuttling a relatively large protein and a snippet of RNA into affected cells. The brain is one of the most challenging organs to deliver, and researchers are struggling to perfect it.
Devinsky assembled a team at NYU Langone Health with expertise in genome editing and neuroscience to conduct preliminary studies of the approach. The researchers were able to use other grants to fund the rest. “We will sell our house if we have to,” Sonam said.
The work has not stopped in India. Rajeev has urged Chakraborty to finish the team’s studies in mice, so that the next person with FENIB will not have to wait as long for a potential treatment. The effort could benefit others with genetic conditions in India, because some of the work will be completed. “We are not really trying as aggressively as we did earlier,” he says. The technology has a lot of potential.
The Sarafs moved back to India in December of 2019. Uditi missed her extended family when she lived in the United States. Then the COVID-19 pandemic struck, and in January 2021, Uditi was hospitalized with severe COVID-19. She spent 20 days in the hospital and her health was never the same, says Sonam. Communication became increasingly difficult for Uditi and she began to pace the house incessantly, rarely even going to sleep.
There was a petition that was submitted to a US foundation for an experimental treatment called antisense therapy for Uditi. The family flew from India to the United States to receive her injections. The trips became traumatic as her ability to understand the world around her declined.
The two brothers went to the institute to talk with Chakraborty and the chemist who had been working with him on the Sickle-cell project.
India has the highest rate of the condition in the world and most of the people are from impoverished communities. Chakraborty and his colleagues hoped to develop a therapy that could be produced and administered at a fraction of the price that is charged in the United States, if not less.
An Indian project to make vaccines for disease control in the era of COVID-19: The race to save one woman’s life
Still, that was not the end of their challenges. AAV genomes can carry only an extra 4,700 DNA bases, but the gene that codes for the enzyme needed in base editing is longer than that. In order to make the cargo fit in two different viruses, a group of students and a professor worked together to create new sequences that would allow the two pieces to be duplicated inside a cell again. The team would inject both viruses at the same time.
It was not the first time that he was swayed by a personal appeal, as he found two women waiting outside of his office a few years before he met Uditi. They would not leave, the women said, until he committed to finding a treatment for their young sons’ illness, a genetic condition called Duchenne muscular dystrophy, which can be fatal. He was unable to say no to the women, who had pledged to raise funds. He has worked on the project and grown close to the families since then.
India is known for making complex drugs on a budget. During the COVID-19 pandemic, Indian manufacturers cranked out millions of doses of vaccines. The country is making a Malaria vaccine at a lower cost than it does in Europe, while it is developing cell and gene therapies used in cancer treatments for less than those in the United States.
Source: Hope, despair and CRISPR — the race to save one woman’s life
Injecting AAV9 into Uditi’s Brain: The Case of a Patient Known as a “Go-getter”
He was leading Uditi’s project. Riya, who was a PhD student in his lab, says that he is a go-getter kind of person. He doesn’t mind who he needs to ask to get something done.
The team mapped out all of their experiments to make sure they didn’t get interrupted. Supply disruptions can delay projects by weeks or months in India. Everything had to be ordered in advance and Maiti worked hard to get the supplies there. He says that time was more valuable than anything else.
Rauthan generated stem cells from samples of Uditi’s blood. Then, she and her colleagues coaxed those cells to become neurons, and used base editing on them in the lab.
Ghosh worked on preparing the AAV that would be used to transport the CRISPR components into Uditi’s neurons. The team needed to determine which strain of AAV would work best — some strains could trigger inflammation in the brain. Ghosh’s lab tested several types of AAV in mice, to find out which one caused the least amount of inflammation and how best to administer it. The team decided to inject the AAV9 into Uditi’s brain.
The news was hard for others in the lab. Chakraborty says that clinicians may become hardened. We don’t have that experience. We were feeling agony.”