Telemedicine to Increase Access to Abortion Medication: The Case of Miscarrying, Misoprostol and Misoprenol
It’s not clear if many or most drugstores in the country, or some of them in red states, will increase access to medication abortion in ways that meaningfully increase abortion access.
Clinics in Colorado, Illinois and New York have seen more patients as women travel out of state for abortions. But the shift to telemedicine makes sense for practical reasons. First, having an abortion with pills at home, which has the physical effects of miscarrying, is as safe and effective in the first trimester as going to a clinic.
The FDA’s pharmacy certification for mifepristone requires pharmacies to track shipments and to keep records of prescribers, recipients and lot numbers of each drug dispensed. This “inhibits the creation of a secondary distribution network for this drug,” Grossman said, such as if people in a state with access send the drug to those in abortion-restriction states.
Andrea Miller, president of the advocacy group National Institute for Reproductive Health, praised the FDA’s changes, calling them an “important step forward” in terms of increasing access to abortion medication – but she said there is “an unfortunate reality.”
The medications can be taken for up to 11 weeks after the first day of a last menstrual period. Telehealth prescriptions are an option in some states, or a person could travel to a state where abortion is legal to get the pills.
According to the Guttmacher Institute, more than half of abortions in the US in the year 2020 will be done with medication, more than surgical procedures.
Following the reversal of Roe v. Wade last year, the USPS sought the advice of the OLC on whether federal law prohibits the mailing of mifepristone and misoprostol.
Attorney General Garland issued a statement promising to work with the FDA and other federal agencies in order to protect access to such drugs which some states have sought to ban.
The Biden administration partially implemented the change last year, announcing it would no longer enforce a long-standing requirement that women pick up the medicine in person. There is an update to the drug’s label that will allow more retail pharmacies to sell it, provided they complete a certification process.
Two drugmakers that make brand-name and generic versions of abortion pills requested the latest FDA label update. The agency requires a company to have an application filed before they modify restrictions on drugs.
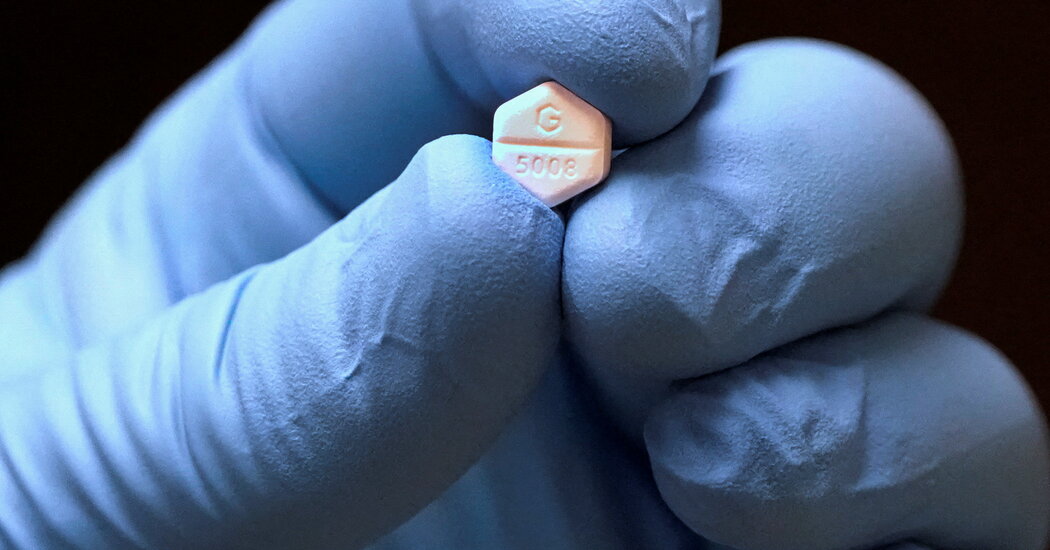
The FDA in 2000 approved mifepristone to terminate pregnancies of up to 10 weeks, when used with a second drug, misoprostol. Mifepristone is taken first in order to dilate the female’s reproductive tract and block the hormones that build up during a baby’s development. The uterus contracts after taking misoprostol for 24 to 48 hours.
Bleeding is a common side effect, though serious complications are very rare. The FDA says more than 3.7 million U.S. women have used mifepristone since its approval.
In the case of excessive bleeding prescribers need to have training so that they can provide emergency care. The pills need certification in order to be distributed in the pharmacy.
The FDA’s Adjustment to REMS: Can It Really Affect Abortion Access in States That Are Banned or Restricted?
It’s not likely that the issue of abortion will have an impact on states where abortion is banned. I don’t think there will be a real effect.
It’s not clear which other pharmacies will seek certification or what impact it will have on abortion access in places where it’s banned or restricted.
In a statement Wednesday, Walgreens said it is “working through the registration, necessary training of our pharmacists, as well as evaluating our pharmacy network in terms of where we normally dispense products that have extra FDA requirements and will dispense these consistent with federal and state laws.”
Honeybee Health was the first digital pharmacy to sell and ship abortion drugs during the Pandemic. We are proud to partner with the majority of telemedicine abortion providers in the US and to work closely with our manufacturer to help set the high standards required for certification in response to the FDA’s adjustment to the REMS program,” the Facebook post says. REMS is the Risk Evaluation and Mitigation Strategy program.
It may be difficult for many pharmacies to decide if they want to get certification.
The Guttmacher Institute’s Elizabeth Nash stated that abortion is still banned in places where it was restricted before the FDA update.
We do not believe that anyone should have to travel that way, and there are a lot of very smart lawyers who are looking at the question of how they can be incorporated into drugstores and pharmacy chains.
He asked, “Would a state that was prosecuting somebody for diversion have access to those records? Because if they do, then that is a disincentive to providing it to people in states that are banning it.”
The FDA’s actions are under scrutiny by the US Supreme Court in the current political environment, and it’s not certain if they’ll allow the FDA to pre-EMPIRE state laws that restrict abortion access.