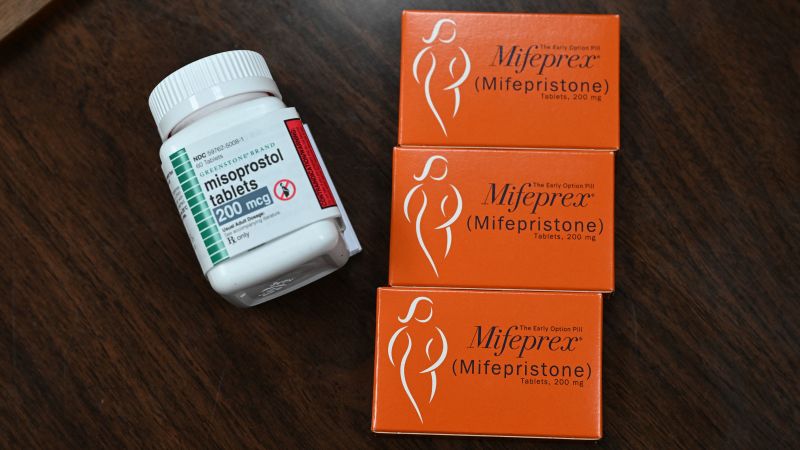
A Facebook Post by Honeybee Health on Abortion with Misoprostol or in Combination with Mifepristone
The FDA said on the website that if a person has a doctor’s prescription, a pharmacy certified by the FDA can offer it directly to them.
The American University Washington College of Law professor said it was the first time an outpatient pharmacy was allowed to sell the drug.
If a state restricts abortion, then medication abortion is unavailable. I think we will see how this tension plays out, between the authority of the FDA and the state laws. “We may see some court cases around this very issue as to FDA’s authority and state law.”
Moseson was the first author of a study published last year2 in The Lancet Global Health that looked into the effectiveness and safety of medication abortion with misoprostol alone or in combination with mifepristone. The study, which followed 961 people who self-managed abortions in Argentina and Nigeria, found that 99% of those who used misoprostol alone had a complete abortion without needing surgical intervention. Among those who used the combined regimen, the rate was 94%.
In a statement Wednesday, Walgreens said it is “working through the registration, necessary training of our pharmacists, as well as evaluating our pharmacy network in terms of where we normally dispense products that have extra FDA requirements and will dispense these consistent with federal and state laws.”
“At the onset of the pandemic, Honeybee Health quickly became the first digital pharmacy to supply and ship medication abortion. The company is proud to partner with most of the telemedicine providers in the US and is working closely with its manufacturer to set the high standards required for certification in response to the REMS program, according to a Facebook post. REMS stands for Risk Evaluation and Mitigation Strategy.
What can states do about Mifepristone if the FDA moves forward or what does it take to enforce their abortion laws? An attorney’s opinion
It will take some time to decide whether or not to go through the certification process, which may be complicated.
Any kind of pharmacy certification is normally needed for mifepristone, but it’s not what they’re used to. “And this particular pharmacy certification regime seems much more onerous than one would expect for a random drug with a similar safety profile.”
She said that it was not clear how the FDA move would affect states’ ability to enforce their abortion restrictions. It is not clear whether someone who lives in a state with abortion restrictions can use a service to get a prescription for Mifepristone and have it delivered through the mail.
abortion is still banned in places where it was banned before the FDA update, said Elizabeth Nash, a policy associate of state issues at the Guttmacher Institute.
“We don’t believe that anyone should be forced to travel in that way, and certainly, as this moves forward, there are a lot of very smart lawyers who are looking at the question of how they’ll be incorporated into drugstores and pharmacy chains, and where that can happen – and how these different federal and state provisions interplay,” she said.
He asked, “Would a state that was prosecuting somebody for diversion have access to those records? Because if they do, then that is a disincentive to providing it to people in states that are banning it.”
Whether states can enforce restrictive abortion laws against people who “provide, facilitate access to, or obtain medication abortion” to someone in another state or within a state depends in part on a doctrine known as preemption, under which a state law that undermines the purpose or objectives of federal law cannot be enforced, Litman said.
Here, Nature explains the evidence in the case, what’s on the line and what abortion options will be available to people in the United States if the FDA loses.
According to the FDA, the approval process for mife Pristone took four years but did not involve an accelerated review. They said that the patient who may have suffered the most serious adverse events associated with Mifepristone had not been brought up in the lawsuit. The court filing says that the omission is particularly telling, since it has been over two decades that the drug has been in use.
Some clinical trials have shown that it has a lower success rate, which is a factor why it isn’t often administered on its own. She said that it might be because of the differences between study design and local conditions. In some studies, if after a few days of taking misoprostol the abortion is not complete, participants are offered a surgical abortion. This would then be registered as an unsuccessful use of the drug. In settings where surgical intervention isn’t readily available, the success rates tend to be higher, Moseson says.
If such a decision were to be appealed, it would land in the US Court of Appeals for the Fifth Circuit, “one of the most conservative appellate courts in the country”, according to Allen. If the court upheld the theoretical decision to ban the drug, the FDA could take the case to the Supreme Court.