Email ban on mifepristone: a lawsuit by the Department of Justice asking for an emergency stay of Kacsmaryk’s decision
When a federal judge in Texas ruled that the Food and Drug administration shouldn’t have approved the abortion pill mifepristone in 2000, he agreed with arguments by plaintiffs who oppose abortion rights in ruling that the agency improperly used a process of accelerated approval that didn’t fully assess the drug’s risks and benefits..
On Monday, the Department of Justice asked the Fifth Circuit for an emergency stay of Kacsmaryk’s decision while they hear the case. In their request, Justice Department lawyers argued that “the district court upended decades of reliance by blocking FDA’s approval of mifepristone and depriving patients of access to this safe and effective treatment, based on the court’s own misguided assessment of the drug’s safety.”
There is no idea what will happen if the email ban stays in place. Even if virtual clinics want to keep dispensing the pills, they may run into an issue with the two major US manufacturers, Danco Laboratories and GenBioPro. David Cohen, a law professor at Drexel University, says that if the Biden administration issues an enforcement discretion notice, the FDA can allow mail-order pharmacy to make certain purchases of prescription drugs.
Since the Supreme Court overturned Wade, reproductive healthcare has become more prominent in virtual abortion clinics. Before that decision, virtual abortion clinics accounted for 4 percent of abortions in the US; after the decision, the number rose to 11 percent, according to a study from the Society of Family Planning.
The Danco Laboratories vs. the Department of Justice: When will the 5th Circuit intervene? A letter to the FDA complaining about the district court’s lawless opinion
We don’t know if the Justice Department or the drug manufacturer Danco Laboratories will ask the Supreme Court to intervene. The 5th Circuit did not act by noon on Thursday and the DOJ would have to go to the high court.
Both the judges who voted to tighten restrictions and the one who did not are appointees of Donald Trump. Bush was the president who appointed the third judge, Catharina Haynes. She said she would have put the lower court ruling on hold entirely temporarily to allow oral arguments in the case.
The judge said she would have granted the expedited appeal but would have issued an administrative stay on Kacmsaryk’s ruling – a temporary hold that would have lasted a “brief period of time” – and deferred the question of whether it should be frozen longer term to the judges hearing the expedited appeal.
Otherwise, they wrote, “the district court’s lawless opinion will empower any plaintiff to grind drug approvals to a halt, disrupting patients’ access to critical medicines. That outcome would chill crucial research and development, undermine the viability of investments in this important sector, and wreak havoc on drug development and approval generally, causing widespread harm to patients, providers, and the entire pharmaceutical industry.”
Jim C. Stansel, the group’s executive vice president and general counsel, sent a letter to the FDA complaining about court substituting expert opinion for the approval of certain drugs.
Mifepristone has been used by millions of women over the past 23 years, and complications from mifepristone occur at a lower rate than problems in wisdom teeth removal, colonoscopies and other routine procedures, medical groups have recently noted.
After the US Supreme Court decided to overturn Wade, many experts predicted that there would be conflicts with the federal agency if drugs are allowed in states other than those where they are forbidden by the federal agency.
The governors of Massachusetts and Washington said their states had begun stocking up on medication in the event that access is disrupted. California Gov. Gavin Newsom and New York Gov. Kathy Hochul say their states are stockpiling tens of thousands of doses of misoprostol.
Reply to the “Comment on ‘Access to Mifepristone” by T.C. Rice’s Motion”
The motion the Justice Department filed Monday asked Rice to clarify his ruling, due to the fact that there is “tension” with Kacsmaryk’s nationwide injunction.
There are contingency plans in place for US providers who could lose Mifepristone. Medication abortions typically consist of two pills: mifepristone and misoprostol. Maternal hormones are required for pregnancies to continue, and Mifepristone works by blocking the hormones. The abortion pill, as it is commonly referred to, is actually Mifepristone that causes the uterus to expel fetal tissue. And as misoprostol is not subject to the recent rulings, there is a possibility that these companies will begin offering misoprostol on its own if manufacturers cut off access to mifepristone. This situation is not ideal as the combination of pills produces the best results and it can cause additional problems like pains and nausea. But for providers determined to keep helping patients, it’s better than nothing.
After conflicting legal rulings triggered widespread uncertainty about the future of abortion pill access in the United States, both US-based telehealth providers and overseas pill-by-mail sellers want to make one thing clear: They’re here to stay.
These companies are moving fast to ensure they are still able to operate legally without interruption, as they are preparing for increased restrictions. In the states where Hey Jane and Choix used to work, they are still offering the pills by mail.
Over 500 pharmaceutical executives and researchers signed an open letter earlier this week, declaring that a decision to side with the conservatives in curtailing access to the drug would result in uncertainty for the entire biopharma industry. Dr. Bourla is the CEO of Pfizer, and executives from industry giants like Bayer and Mercurial were also signed.
As the lawsuit was being considered in the lower court, industry representatives stayed away, but other medical groups weighed in, like the American Medical Association.
“Defendants have not shown that plaintiffs are unlikely to succeed on the merits of their timely challenges,” the three-judge panel wrote. An injunction will take effect this weekend but there is no chance of an urgent intervention by the Supreme Court.
“Industry members are wondering, well, if a judge can do that, what else can’t a judge, perhaps with an ax to grind, do?” he said in an interview with NPR’s Morning Edition before the appeals court ruled.
The FDA’s decision to approve RU-486: All cards are on the table for the litigation if there is a problem with it, says law professor Allison Whelan
The time and cost that brings new drugs to market is already high. To research and develop a new medical product can cost hundreds of millions of dollars and years of clinical trials.
It’s not worth the risk of financial loss to invest in drugs if an FDA approval can be revoked in whole, or in part, at any time by a judge.
Allison Whelan is a law professor at Georgia State University. She explained that this was not about safety and efficacy reasons but about stopping vaccines that you do not agree with.
The litigation could become a competitive tool if left unaddressed. She said that a competitor could keep a product off the market if they disagreed with the FDA’s decisions about a drug.
Harvard’s Sarpatwari said that he expected the pharma industry to be aggressive in lobbying congress and trying to preserve the FDA approval process. “I think that all cards are on the table in terms of what industry may do,” he said.
Dr. Joshua Sharfstein is a vice dean at the Bloomberg School of Public Health and was a deputy Commissioner for the FDA. This was supported by advisory committees. It had the full support of major professional associations, and it retained that support after millions of women have received the treatment.”
The drug became approved in China, the United Kingdom, and Sweden in the early 1990s. In 1999 there were more countries that approved the drug.
Sharfstein says there’s a problem if there is a problem with this medicine. “Because this is very much in line with what FDA does and has the full support of the medical community.”
The agency used a part of the approval that made it possible to add safety restrictions, such as requiring physicians who give the pill to be able to diagnose ectopic pregnancies.
“There are people who have wanted RU-486 to be pulled off the market since the day it was approved,” then-Rep. Henry Waxman, a Democrat from California, said at the time. They didn’t want it to be approved. I respect their judgment because they are very strongly against an abortion, whether it be by RU-486 or by a medical procedure. But that is not the issue of safety and it is not an issue of science and it is not an issue of data.”
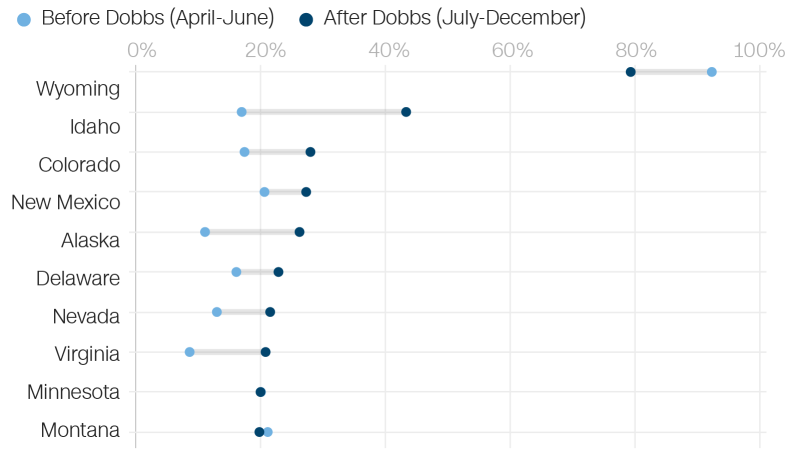
Before and after the Dobbs ruling of any state, Wyoming had the highest rate of live abortions. In the months following the decision about abortion, the number of abortions that were done via the internet dropped from almost all of them.
The Supreme Court is expected to respond by the end of the week to a Justice Department request that it block a federal appeals court decision limiting access to abortion drugs.
The government requested an administrative stay to preserve the status quo while the court considers the request. Portions of a Texas district court’s order that limits the drug would otherwise take effect at 1:00 a.m. ET on Saturday, April 15.
In November of 2022, anti- abortion groups filed a lawsuit in Texas against the FDA for approving the drug in 2000.
The final ruling from the appellate panel led the DOJ to appeal even higher to the Supreme Court in hopes of ensuring access to the drug is fully restored.